Medicine and Health
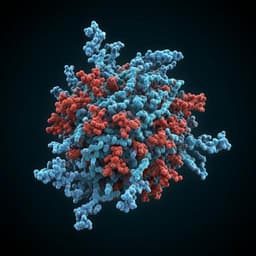
Structural insights into the modulation of coronavirus spike tilting and infectivity by hinge glycans
D. Chmielewski, E. A. Wilson, et al.
This groundbreaking study by David Chmielewski, Eric A. Wilson, and colleagues unveils the intricate structure and dynamics of coronavirus spikes, shedding light on their infectivity. Utilizing advanced techniques like cryo-electron tomography and mass spectrometry, the research pinpoints critical glycosylation roles, offering novel insights into potential therapeutic targets for HCoV-NL63.
Related Publications
Explore these studies to deepen your understanding of the subject.