Medicine and Health
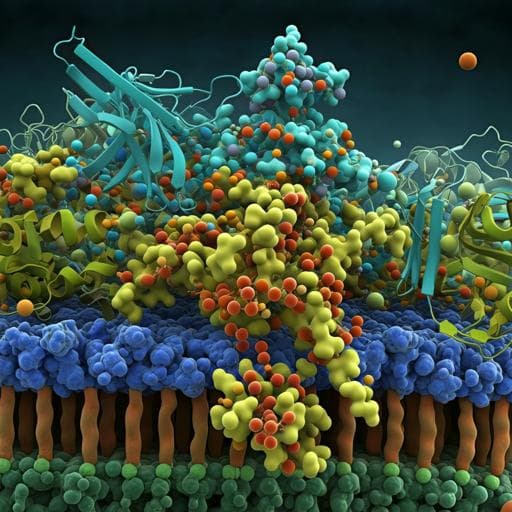
MFRP is a molecular hub that organizes the apical membrane of RPE cells by engaging in interactions with specific proteins and lipids
A. Tworak, R. Smidak, et al.
Discover how the absence of Membrane frizzled-related protein (MFRP) in the retinal pigment epithelium (RPE) leads to significant ocular issues like hyperopia and retinitis pigmentosa. This research, conducted by a team at the Gavin Herbert Eye Institute, unveils MFRP's critical role in retinal health and offers insight into potential gene therapy outcomes.
Related Publications
Explore these studies to deepen your understanding of the subject.