Medicine and Health
A wireless battery-free eye modulation patch for high myopia therapy
T. Zhong, H. Yi, et al.
High myopia, often defined as refractive error worse than −5.0 D, is projected to affect about 20% of the global population by 2050. Many cases progress despite standard optical or refractive surgical corrections, leading to pathological changes in the retina, choroid, and sclera and elevating blindness risk. Two main clinical strategies exist: posterior scleral cross-linking (chemical strengthening of sclera) and posterior scleral reinforcement (PSR, mechanical support). Both face limitations, including difficulty exposing the posterior pole, inefficient light delivery with optical fibers, procedure complexity, and dependence on surgeon skill with limited intraoperative measurement precision. The study posits that a compact, wirelessly powered, battery-free implant capable of both axial length (AXL) modulation and posterior scleral reinforcement via drug delivery and light-induced collagen cross-linking could overcome these challenges, enabling precise AXL correction and durable strengthening to prevent progression and relapse of high myopia.
Prior approaches to progressive high myopia include posterior scleral cross-linking (riboflavin with UV or blue light) to increase scleral rigidity and posterior scleral reinforcement (PSR) using materials to support the posterior pole. Animal studies suggest cross-linking strengthens posterior sclera, while PSR has shown safety and efficacy over decades, improving macular conditions (e.g., macular splitting, detachment) and reducing further surgeries. However, posterior scleral cross-linking is limited by the difficulty of fully exposing the posterior pole; fiber-based light delivery suffers from adhesion issues and blood flow obstruction. PSR requires extensive intraoperative exposure, is technically demanding, and lacks precise intraoperative measurement for AXL changes. Emerging microneedle technologies enable targeted ocular drug delivery (cornea, suprachoroidal space, sclera), and battery-free, wirelessly powered implantable microsystems have been developed for neuromodulation and drug delivery. These advances motivate an integrated device that wirelessly powers precise AXL modulation, efficient riboflavin delivery to posterior sclera, and on-demand blue light cross-linking to strengthen tissue.
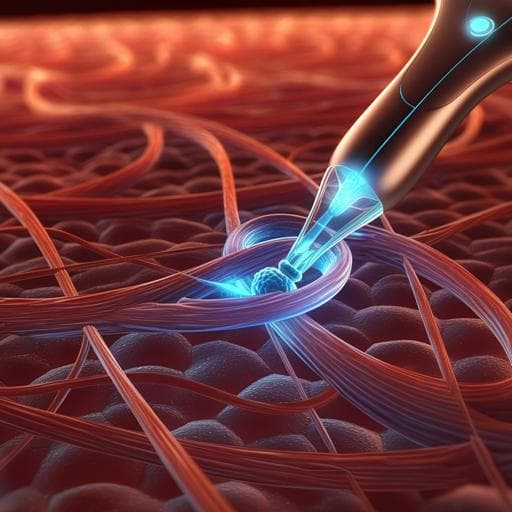
Device design: A multifunctional, flexible, battery-free patch integrates three PZT piezoelectric transducers (3 mm diameter, 1 mm thickness), an electrochemical micro-actuator, a riboflavin-loaded dissolvable microneedle array, three blue µ-LEDs (peak 443 nm) for scleral collagen cross-linking (SCXL), two red µ-LEDs for intraoperative localization, a flexible microfabricated circuit, and PDMS encapsulation. The patch is horseshoe-shaped with a central concavity to avoid the optic nerve, positioned adjacent to the optic nerve over the macular area, secured by three sutured legs. Overall thickness ~4 mm; distance between rear legs 19 mm; mass 0.41 g. The micro-actuator reservoir (radius 7.4 mm) is sealed by a PDMS/SBS bilayer membrane (~440 µm total thickness) and contains 35 µL of 50 mM NaOH electrolyte. Wireless powering and control: An external ultrasound source drives each PZT independently at 633 kHz with adjustable duty cycle (10–100%; typically 30%), converting acoustic to electrical energy. Impedance matching ensures efficient power transfer through gel and ocular tissue. Output is stable across 1–30 mm separation and various angles. PZT1 powers red µ-LEDs for localization; PZT2 powers a rectified DC electrolysis circuit (indicator µ-LED on leg) to drive the micro-actuator; PZT3 powers three blue µ-LEDs for SCXL (indicator µ-LED on leg). In pork tissue depths (7–35 mm), the wireless output is ~7 V and 4–5 mA. Electrochemical micro-actuator: Cu/Au interdigitated electrodes electrolyze aqueous NaOH to generate H2 and O2, increasing internal pressure and expanding the membrane to indent the posterior sclera inward, shortening AXL. Max electrolysis current occurs at ~4.5 V; bubble generation fastest near 3.5 V. Chamber volume optimization determined a minimum 25 µL is required to avoid bubble shielding; device uses 35 µL (chamber height ~2.5 mm). Under ~−5 mA operation, maximum membrane deformation ~870 µm in 200 s (validated experimentally and theoretically). In vitro porcine eye tests show AXL shortening of ~590 µm. Thermal monitoring shows minimal heating: micro-actuator 22.2→23.5 °C in 6 min; PZT 21.9→34.6 °C in 6 min, below body temperature. Microneedle array and SCXL: PVP microneedles (height 400 µm, base diameter 370 µm, 600 µm pitch) loaded with riboflavin (≈4.8 mg per array) fabricated using a PDMS mold cast from a silicon master. Riboflavin fluorescence confirms drug distribution. Mechanical testing shows >2.1 N compressive strength per microneedle at 400 µm height, sufficient to penetrate posterior sclera. Penetration depth into porcine posterior sclera ~84 µm without affecting choroid/retina. Drug diffusion into sclera verified by time-lapse fluorescence (0–30 min). Blue µ-LED intensity under typical current (~9.6 mA at ~10 mm ultrasound distance) is ~32 mW cm−2, aligning with riboflavin’s absorption. Porcine sclera Young’s modulus increased to 40.3 MPa (~136% over control and riboflavin-only groups) after SCXL. Three µ-LEDs arranged around the patch ensure adequate and uniform irradiation of the target area (COMSOL simulations) compared with one or two µ-LEDs. Animal study (rabbits): Ten 3-month-old New Zealand White rabbits (both sexes) were used. Patch implantation was performed on the right eye only; left eye served as untreated reference. Preoperative assessments included IOP, AXL, OCT, and fundus imaging. Under general anesthesia, a 360° conjunctival peritomy and muscle dissection exposed the posterior sclera. The patch apex pressure point was positioned 5–8 mm inferior to the optic disc and horizontally aligned; three legs were sutured to sclera (5-0 Vicryl). After 30 min to allow riboflavin infiltration, the micro-actuator was wirelessly activated for 6 min to modulate AXL, followed by blue µ-LED irradiation for 30 min (1-min breaks every 5 min) to induce SCXL. Postoperative IOP, AXL, OCT, and fundus imaging were repeated. Follow-up lasted up to 22 days. Fabrication and materials characterization: Flexible Cu/PI/Cu substrate patterned for transmission lines and interdigitated electrodes; through-vias (150 µm) electroplated to interconnect layers; Au (75 nm) deposited to protect Cu in NaOH. PDMS encapsulation and reservoir molded via 3D-printed silicone molds; PDMS/SBS bilayer formed after oxygen plasma treatment to enhance adhesion (contact angle reduced from 115.3° to 6°; SBS layer contact angle 80.2%). ATR-FTIR confirmed hydroxylation of PDMS after plasma. Optical transmittance at 443 nm: PDMS 94.29%, plasma-treated PDMS 88.59%, SBS 79.71%. SEM verified smooth, crack-free surfaces and ~390 µm PDMS + ~50 µm SBS thickness. Mechanical tests of microneedles used a TMS-Pro Texture Analyser. Imaging modalities included OCT, fundus photography/angiography, A/B-scan ultrasonography, and Optos wide-field imaging. Histology and safety: H&E, toluidine blue, immunofluorescence (IBA1 for microglia, GFAP for astrocytes), and TUNEL assays assessed tissue responses. IOP monitoring conducted peri- and postoperatively. Statistical analyses used t-tests or one-way ANOVA with p<0.05 considered significant.
- Wireless powering: Stable output through tissues at 633 kHz; ~7 V and 4–5 mA at depths 7–35 mm; current to blue µ-LEDs ~9.6 mA at ~10 mm separation delivering ~32 mW cm−2 irradiance.
- Micro-actuator performance: Membrane deformation ~870 µm within 200 s at ~−5 mA; in vitro porcine eye AXL change ~590 µm. Minimal heating: micro-actuator 22.2→23.5 °C; PZT 21.9→34.6 °C over 6 min; below body temperature.
- AXL modulation in vivo (rabbits): Mean AXL reduction ~1217 µm within ~6 min (n=6; paired t test p=0.0004). OCT B-scans and 3D reconstructions showed posterior pole deformation consistent with AXL shortening (~1030 µm bulge on B-scans). Ultrasound A/B-scan corroborated AXL changes.
- Scleral strengthening: In porcine sclera ex vivo, SCXL increased Young’s modulus to 40.3 MPa (~+136% vs control and riboflavin-only). In rabbits in vivo, posterior sclera Young’s modulus increased by ~151% at day 7 and ~387% at day 22 post-SCXL (n=4 per group; unpaired t tests p=0.0434 and p=0.0059, respectively). H&E indicated denser, more organized collagen with 58.33% reduced porosity vs control.
- Drug delivery: Microneedles penetrated ~84 µm into sclera without affecting choroid/retina; >2.1 N strength per microneedle; progressively increasing scleral fluorescence within 30 min post-application demonstrated riboflavin diffusion.
- Optical safety/efficacy: Scleral/choroidal transmission at ~30 mW cm−2 pre-penetration was ~55.46% (rabbit) and ~15.40% (pig); three-µLED configuration ensured adequate and uniform irradiation for effective SCXL.
- Safety and tolerability: IOP transiently peaked on surgery day (16.6–23.6 mmHg) and returned to ~10.3 ± 2 mmHg by day 3, remaining near baseline over 20 days. No microglial activation (IBA1) or astrocyte reactivity (GFAP), minimal TUNEL-positive cells, no fundus complications (hemorrhage, detachment, edema), and uneventful wound healing over 22 days. Body weight and temperature remained within normal ranges.
The study demonstrates that a compact, wirelessly powered, battery-free patch can both acutely modulate ocular axial length and durably strengthen the posterior sclera, addressing two key drivers of progressive high myopia: excessive axial elongation and scleral laxity. Ultrasound-enabled selective powering of distinct on-board modules allows precise, on-demand control of localization, electrochemical actuation, and photo-induced collagen cross-linking. The electrochemical micro-actuator effectively indents the posterior pole to reduce AXL by ~1.2 mm in rabbits within minutes, translating to a potentially meaningful refractive correction in humans (approx. −2.5 D projected by the authors). The integrated riboflavin microneedle array achieves efficient drug delivery into dense scleral tissue, while blue µ-LEDs provide targeted illumination that significantly increases scleral stiffness (up to +387% at 22 days in vivo), offering reinforcement that may limit relapse after AXL correction. Safety assessments indicate minimal thermal effects, preserved retinal and choroidal structure, stable postoperative IOP, and absence of overt inflammation or neuroglial activation. Collectively, these results support the feasibility and potential clinical value of a wireless, minimally invasive therapy that combines precise biomechanical modulation with tissue reinforcement to prevent or slow progression of high myopia.
This work introduces a fully integrated, wireless, battery-free eye modulation patch that combines ultrasound-controlled electrochemical actuation for rapid AXL shortening with riboflavin microneedle delivery and blue µ-LED-induced scleral cross-linking for sustained biomechanical reinforcement. The system achieved substantial AXL reduction (~1.217 mm) and markedly increased scleral stiffness (up to +387% at 22 days) in rabbits, with favorable safety findings. The approach could reduce surgical complexity, enable precise intraoperative and postoperative control, and potentially lower recurrence of myopic progression. Future research should include larger cohorts, extended follow-up to assess long-term durability and safety, optimization of actuator and illumination parameters for human anatomy, evaluation of dose–response and repeatability, and translational studies to quantify refractive outcomes and functional vision benefits in humans.
The study is limited by a small sample size and a relatively short follow-up period (22 days), precluding definitive conclusions about long-term efficacy, durability of scleral reinforcement, and late adverse events. Translation from rabbit to human eyes requires consideration of anatomical and biomechanical differences. Thermal rise of the PZT during operation underscores the need to limit activation duration. Individual variability in implantation and suture-induced positioning may influence AXL adjustments. Comprehensive visual function outcomes were not assessed.
Related Publications
Explore these studies to deepen your understanding of the subject.