Biology
Structure of the native pyruvate dehydrogenase complex reveals the mechanism of substrate insertion
J. Škerlová, J. Berndtsson, et al.
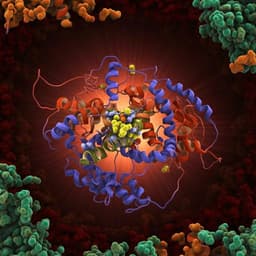
This groundbreaking research reveals the intricate workings of the pyruvate dehydrogenase complex in *E. coli*, showcasing a cryo-EM reconstruction that uncovers the assembly and dynamics of dihydrolipoyl transacetylase. Conducted by Jana Škerlová, Jens Berndtsson, Hendrik Nolte, Martin Ott, and Pål Stenmark, this study sheds light on the active site's substrate shuttling mechanism.
Related Publications
Explore these studies to deepen your understanding of the subject.