Medicine and Health
Structural basis for modulation of human Nav1.3 by clinical drug and selective antagonist
X. Li, F. Xu, et al.
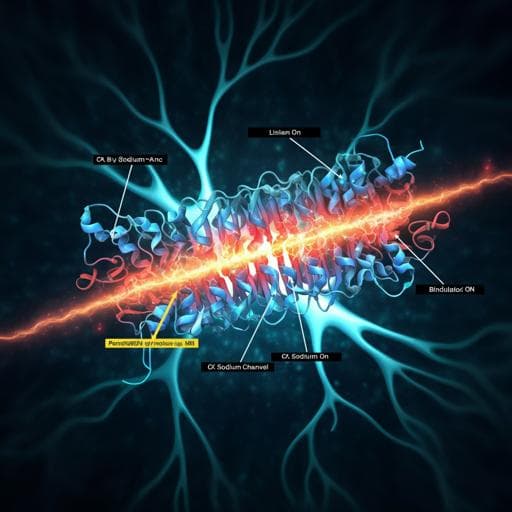
Dive into the fascinating world of voltage-gated sodium channels! This study reveals how NaV1.3 interacts with specific modulators like bulleyaconitine A and ICA121431, shedding light on their unique binding mechanisms. Conducted by esteemed researchers including Xiaojing Li and Feng Xu, these findings are paving the way for targeted therapeutic advancements.
~3 min • Beginner • English
Related Publications
Explore these studies to deepen your understanding of the subject.