Environmental Studies and Forestry
Interfacial assembly of self-healing and mechanically stable hydrogels for degradation of organic dyes in water
G. Yan, Y. Feng, et al.
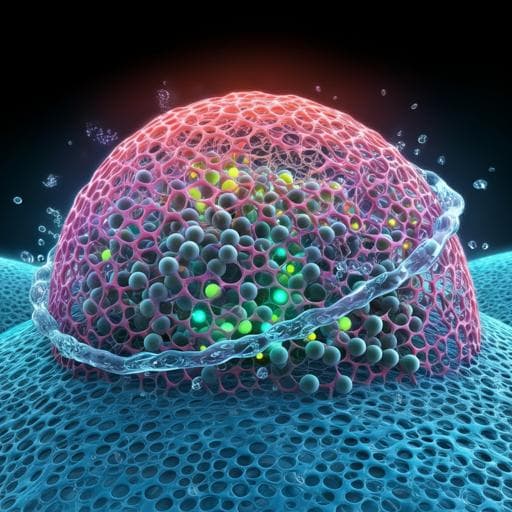
The study addresses the challenge of deploying hydrogels for underwater applications such as water purification and pollutant degradation. Conventional hydrogels suffer from weakened mechanical properties and stability when immersed, particularly due to water’s disruption of van der Waals interactions and the detrimental effects of complex aqueous environments containing metal ions and salts that can deposit and damage structures. Mussel-inspired hydrogels using catechol chemistry (e.g., polydopamine, PDA) improve underwater adhesion and toughness via covalent and noncovalent interactions and dynamic bonds, but pure PDA-functionalized hydrogels may still fall short of practical mechanical demands, and overoxidation of catechol groups can degrade performance. Selective, chemically stable surface membranes are also needed to regulate ion and particle transport and protect hydrogel integrity. The authors propose an integrative approach: a cellulose-based hydrogel incorporating PDA-loaded Pd nanoparticles to sustain a dynamic catechol redox system for durability and self-healing, paired with a covalently grafted graphene oxide membrane to enhance hydrophilicity, selective permeability, and antifouling, thereby enabling efficient, reusable degradation of organic dyes in water.
Prior work has explored hydrogels for underwater uses in tissue engineering, chemical engineering, and biomedicine, including water purification and pollutant adsorption/degradation. Mussel-inspired PDA-based hydrogels enhance underwater mechanical properties due to covalent/noncovalent bonding and dynamic bonds enabling self-healing. However, purely PDA-functionalized hydrogels often do not achieve the necessary mechanical strength for practical use, and overoxidation of catechols deteriorates performance, highlighting the need to control catechol oxidation state. Membrane strategies, such as graphene oxide-based selective layers, can improve selective permeation, hydrophilicity, and antifouling, potentially protecting hydrogel structures from metal ions and salt deposition. The literature suggests combining catalytic performance, long-term mechanical stability, and selective permeation in an integrated system could meet high-efficiency underwater application requirements, yet practical demonstrations have been limited.
Materials: Reagents included sodium periodate, sodium chlorite, HCl, dopamine hydrochloride (DA), sodium alginate (SA), acrylic acid (AA), graphene oxide (GO, 1 wt%), poly(N-isopropylacrylamide) (PNIPAm), ammonium persulfate (APS), palladium chloride (PdCl2), sodium borohydride (NaBH4), phosphate buffers, dialysis bags, and ethanol. Deionized water was used for preparation; tap water was used for dye degradation tests.
- Preparation of cellulose nanofibrils (DACO): Bamboo pulp produced by the CAOSA process was mechanically refined. Oxidation with NaClO2 at pH 5.8 (PBS) for 24 h followed by NaBH4 reduction for 1 h yielded a stable emulsion that was dialyzed for 2 days to obtain soluble dialdehyde cellulose (DACO) suspensions (2.3 wt% by freeze drying). DACO possessed abundant carboxyl (9.64 mmol g−1), aldehyde (5.29 mmol g−1), and hydroxyl groups.
- Preparation of DACO-PDA and Pd-loading (DACO-PDA@Pd NPs): DACO was mixed with DA for 12 h at room temperature to form DACO-PDA, followed by NaBH4 treatment and dialysis. PdCl2 solution (20 mM in HCl) was added dropwise to DACO-PDA at 80 °C for 2 h. Negatively charged carboxyl groups attracted Pd(II) electrostatically, while aldehyde and hydroxyl groups reduced Pd(II) to Pd(0), yielding Pd nanoparticles (15–40 nm) immobilized on DACO-PDA fibrils. XRD peaks at 40.1°, 46.6°, 68.9°, and 82.5° assigned to Pd (111), (200), (220), (311)/(222) and XPS confirmed Pd deposition. TEM/SEM showed well-dispersed, single-crystalline Pd NPs (codirectional lattice fringes).
- Hydrogel formation: A homogeneous mixture of AA, SA, APS, and DACO-PDA@Pd NPs solution was prepared, injected into molds, and polymerized at 60 °C for 2 h to afford p(DACO-DA@Pd NPs-co-AA-SA) (DPAS) hydrogels. Mechanism: Pd NPs on DACO-PDA catalyzed redox conversion of catechol to semiquinone and quinone/hydroquinone species, generating free radicals (confirmed by ESR) to initiate AA polymerization and enabling reversible quinone–catechol chemistry that forms covalent/noncovalent cross-links. Carboxyl and hydroxyl groups provided hydrogen/ionic bonding sites; Pd NPs served as nanoreinforcement.
- Surface modification with PNIPAm–GO membrane: Hydrogels (5 × 1 × 0.1 cm, ~0.5 g) were immersed in aqueous GO with PNIPAm and APS at ~25 °C for ≥12 h, then polymerized at 60 °C for 2 h. The resulting PNIPAm-co-GO membrane (P-GOM) was covalently grafted onto hydrogel surfaces (DPAS@P-GOM) and dialyzed 48 h. FT-IR (1100–1500 cm−1 new peaks, C=C fracture on GO) verified modification.
- Characterization: FT-IR for functional groups; TEM/SEM for morphology; EDX for elemental analysis; XRD for crystallinity; UV–vis for Pd(II) reduction monitoring and catalytic activity; ESR for radical detection; mechanical and adhesion tests via Instron 4465; LC–MS/GC–MS for dye degradation products; ICP–MS for Pd leaching (LOD 0.4 ppt).
- Dye degradation tests: DPAS@P-GOM hydrogels were tested for catalytic reduction of Congo red (CR) and methylene blue (MB) in tap water with NaBH4. Standard batch: 10 mL of 30 mg L−1 dye + 5 mL of 40 mg L−1 NaBH4, total 15 mL, stirred. Time-dependent UV–vis absorbance (CR: 250–600 nm, λmax 495 nm; MB: 525–725 nm, λmax 665 nm) tracked discoloration. Controls without hydrogel or without Pd NPs/NaBH4 were performed. Reusability: Five successive cycles using the same hydrogel; after each run, the solution was returned and stirred to continue; hydrogels were also tested in a gravity-driven funnel setup. Pd leaching was quantified after 5 and 50 cycles.
- Hydrogel structure and radical generation: Only DACO-PDA@Pd NP solutions generated sufficient quinone/semiquinone radicals to trigger free-radical polymerization (ESR evidence). Pd NPs (15–40 nm) were uniformly immobilized on DACO-PDA; XRD/XPS confirmed Pd(0).
- Mechanical performance: Incorporation of DACO-PDA@Pd NPs significantly enhanced compressive and tensile strengths relative to pure PAA/SA hydrogels. DPAS hydrogels showed nearly unchanged load–unload compression curves over 5 cycles, indicating good recoverability. Tensile stress–strain increased with Pd NP content, maximizing at formulation DP0.05A2S0.4. Under load, hydrogels exhibited layered stacking (compression) and interwoven microfibrillar structures (tension) consistent with strong cross-linking.
- Adhesion: Adhesion strength to pigskin increased with DACO-PDA@Pd NP content, reaching 34.7 ± 2.6 kPa at 0.05 wt% DACO-PDA@Pd NPs, and remained robust over 15 cycles.
- Self-healing: Manually fractured hydrogels self-healed at room temperature within 2 h; tensile testing of healed samples showed ~90% recovery of tensile properties after 2 h.
- Surface wettability and protection: Water contact angle (WCA) decreased from 74.9° (DPA) to 33.5° (DPAS) and to 0° upon GO surface modification (DPAS@P-GOM), indicating superhydrophilicity. The P-GOM layer provided a dense, hydrophilic, antifouling coating that regulated permeability and rejected metal ions/large particles, protecting the hydrogel from salt deposition and structural damage.
- Dye degradation efficacy: With DPAS@P-GOM and NaBH4, CR discoloration reached ~98% in ~20 min; without hydrogel, discoloration was minimal after 60 min. MB was degraded within ~3.5 min under identical conditions. Mechanism: Pd NPs served as electron relay sites, transferring electrons from BH4− to dye molecules; azo bonds (CR) were reduced to imines, yielding low-toxicity, colorless small molecules (products confirmed by LC–MS/GC–MS).
- Reusability and stability: For CR, reduction time increased slightly from 20 min (1st cycle) to 23 min (5th cycle) with sustained high efficiency; for MB, time increased from 3.5 to 4 min over 5 cycles with >99% efficiency each cycle. DPAS@P-GOM hydrogels showed no obvious morphological changes after 5 cycles (SEM, FT-IR), whereas uncoated DPAS hydrogels were seriously damaged, attributed to salt deposition. Pd leaching was below ICP–MS detection after 5 and even after 50 cycles. Removing Pd NPs slowed degradation; reintroducing Pd restored rates, confirming Pd’s catalytic role.
The integrated hydrogel design addresses the key barriers for underwater catalytic applications. Internally, DACO-PDA@Pd NPs establish a dynamic catechol redox environment that promotes reversible quinone–catechol bonding, enabling high toughness, elasticity, and rapid self-healing, thereby maintaining mechanical integrity during repeated loading and operation. Pd NPs function as both radical initiators for polymerization and as catalytic sites for electron transfer from BH4− to dye molecules, achieving rapid degradation of both anionic (CR) and cationic (MB) dyes. Externally, the PNIPAm–GO membrane confers superhydrophilicity and selective permeation, facilitating water and organic passage while intercepting metal ions and larger particulates that would otherwise foul or structurally compromise the hydrogel. This protective layer prevents salt deposition and preserves the hydrogel framework, enabling stable multi-cycle use with negligible Pd leaching. Together, these mechanisms yield a reusable, mechanically stable platform capable of efficient dye degradation in aqueous environments, directly addressing the initial research goals.
The authors present a cellulose-based, mussel-inspired hydrogel incorporating polydopamine-supported Pd nanoparticles and a covalently grafted PNIPAm–GO surface membrane. The system exhibits high mechanical strength, strong adhesion, rapid self-healing (~2 h, ~90% tensile recovery), superhydrophilicity (WCA ~0°), effective catalytic degradation of CR (~98% in ~20 min) and MB (~3.5 min) with NaBH4, multi-cycle stability with minimal performance loss, and negligible Pd leaching even after extensive reuse. The dynamic catechol redox chemistry underpins mechanical robustness and self-repair, while the GO-based membrane ensures selective permeability and protection from metal ions and salt-induced damage. This integrative approach offers a promising route for durable, reusable hydrogel-based water purification. Potential future directions include extending the platform to broader pollutant classes (e.g., pharmaceuticals, heavy metal complexes), exploring alternative green reductants or catalyst systems, scaling membrane–hydrogel architectures for continuous flow operation, and long-term fouling studies in real wastewater matrices.
The degradation experiments focused on two model dyes (CR and MB) in tap water with added NaBH4 as a sacrificial reductant, which may not fully represent the complexity of real wastewater (variable pH, competing species, organic load, and oxidants). Performance beyond five cycles was indicated for Pd leaching but not fully quantified for catalytic efficiency over many more cycles. Mechanical data are comparative but lack exhaustive quantitative benchmarking across all formulations. Long-term stability under continuous flow, biofouling resistance, and regeneration strategies without NaBH4 were not evaluated. Mapping selective ion rejection across a range of ions and salinities, and the membrane’s durability under harsh conditions, warrants further study.
Related Publications
Explore these studies to deepen your understanding of the subject.