Biology
Enzymatic kinetic resolution of desmethylphosphinothricin indicates that phosphinic group is a bioisostere of carboxyl group
D. D. Biase, F. Cappadocio, et al.
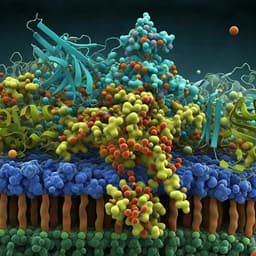
Discover how Escherichia coli glutamate decarboxylase (EcGadB) transforms a unique phosphinic analog of glutamate into a metabolite with promising implications for prodrug design. This fascinating research was conducted by Daniela De Biase, Francesca Cappadocio, Eugenia Pennacchietti, Fabio Giovannercole, Antonio Coluccia, Jouko Vepsäläinen, and Alex Khomutov.
~3 min • Beginner • English
Related Publications
Explore these studies to deepen your understanding of the subject.