Engineering and Technology
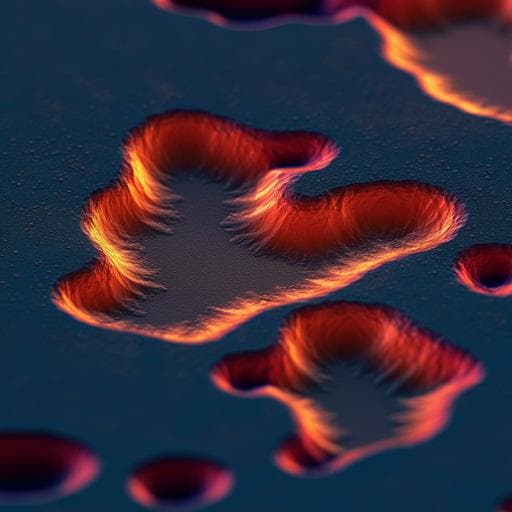
Directly monitoring the shift in corrosion mechanisms of a model FeCrNi alloy driven by electric potential
T. Liu, C. Li, et al.
This groundbreaking study by Tingkun Liu, Cheng-Han Li, Matthew Olszta, Jinhui Tao, and Arun Devaraj delves into the electrochemical corrosion kinetics of austenitic alloys, revealing insights into pit dissolution mechanisms and the influences of electric bias. Discover how these dynamics can enhance our understanding of corrosion across various materials!
Related Publications
Explore these studies to deepen your understanding of the subject.