Medicine and Health
Broad ultra-potent neutralization of SARS-CoV-2 variants by monoclonal antibodies specific to the tip of RBD
H. Ma, Y. Guo, et al.
SARS-CoV-2 has caused more than 200 million confirmed infections and 4.5 million deaths in the first eighteen months of the COVID-19 pandemic, with ongoing transmission. While vaccines offered hope for control, antigenic evolution—particularly mutations in the spike (S) protein’s receptor-binding domain (RBD) that mediates interaction with ACE2—has altered viral immunogenicity, enabling immune escape and complicating control efforts. Neutralizing antibodies targeting the RBD have been effective, but substitutions in RBD can reduce their efficacy. Variants of concern (VOCs) such as Alpha, Beta, Gamma, and Delta carry key RBD substitutions associated with increased transmissibility and reduced susceptibility to neutralization. In this context, the authors sought to discover neutralizing monoclonal antibodies with broad and potent activity against globally impactful variants, especially Delta. By isolating RBD-positive B cells from convalescent individuals, they identified and characterized the antibody 2G1, which efficiently neutralized multiple VOCs and was subsequently evaluated biophysically, biologically, structurally, and in preclinical models.
The paper highlights the evolving threat posed by SARS-CoV-2 variants with RBD substitutions that affect antibody binding and vaccine efficacy. Alpha (B.1.1.7) bears N501Y and shows 50–100% higher transmissibility; Beta (B.1.351) carries K417N, E484K, N501Y and exhibits marked resistance to convalescent and vaccine sera, with E484K linked to immune escape; Gamma (P.1) shares E484K and N501Y with K417T and reduces susceptibility to antibody treatments and vaccine protection; Delta (B.1.617.2) includes L452R and T478K in RBD and displays broad resistance to S-directed antibodies, infecting individuals previously exposed to Beta/Gamma. Additional emerging variants (e.g., Lambda C.37 and Mu B.1.621) raise concerns about further compromise of vaccines and antibody therapeutics. This landscape underscores the need for broadly neutralizing antibodies targeting conserved or mutation-tolerant epitopes on the RBD.
- Donor samples and B cell isolation: Blood from 20 convalescent individuals infected in February 2020 was collected. PBMCs were enriched, and RBD-specific B cells were sorted using fluorescence-labeled recombinant WA1/2020 RBD by flow cytometry (7AAD-/CD19+/CD27+/IgG+/RBD+).
- Antibody cloning and initial screening: >1200 single B cells were isolated; >500 paired IgG antibody genes were cloned via single-cell PCR (375 kappa, 174 lambda). ELISA pre-screening against RBD identified 143 RBD-binding antibodies. Pseudovirus-based screening was then used to prioritize neutralizing candidates across serial dilutions (10 µg/mL to 10−3 µg/mL), identifying 2G1 with IC50 < 0.01 µg/mL.
- Binding and kinetics: ELISAs measured binding to WA1/2020 RBD-mFc and S trimer (EC50 0.016 µg/mL and 0.135 µg/mL, respectively). Surface plasmon resonance (SPR) assessed Fab-RBD kinetics: association rate ka = 2.55 × 10^5 M−1 s−1; dissociation rate kd = 1.05 × 10−3 s−1; equilibrium Kd = 0.41 nM.
- ACE2-blocking assays: Competitive ELISAs quantified 2G1 inhibition of ACE2 binding to WT and single-point RBD mutants (N439K, Y453F, E484K, N501Y), and to S-trimers of WA1/2020 and VOCs (Alpha, Beta, Gamma, Delta). Reported IC50 values were derived from dose-response curves.
- Variant binding kinetics: SPR measured 2G1 Fab binding to S-trimers from WA1/2020 and VOCs (Alpha, Beta, Gamma, Kappa, Delta) to obtain real-time association/dissociation and Kd values.
- Neutralization assays: Pseudovirus neutralization used luciferase-reporter pseudoviruses (titers >1×10^7 TU/mL) representing D614G, Alpha, Beta, Gamma, Delta, Cluster 5. Live-virus neutralization used Vero E6 cells exposed to WA1/2020 and VOCs, with threefold serial dilutions of 2G1; after 3 days, cytopathic effect/plaque counts yielded IC50 values.
- In vivo efficacy: Antiviral activity of 2G1 was tested in ACE2 transgenic mice and rhesus macaques. In mice, animals were challenged with 100× LD50 of WA1/2020, Beta, or Delta, then treated with 2G1 at 20, 6.7, or 2.2 mg/kg versus PBS. Rhesus macaques were similarly challenged and treated; outcomes included clinical observation, viral burden, and safety assessments.
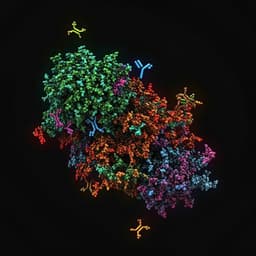
- Structural studies: Cryo-electron microscopy characterized the 2G1 epitope on RBD, interaction interface, and biophysical basis (e.g., hydrophobic interactions) underpinning high-affinity binding and ACE2-blocking.
- Mutational tolerance: Mutagenesis experiments around and within the 2G1 epitope assessed the impact of substitutions on binding and neutralization to evaluate resilience to emerging variants.
- Discovery and potency: 2G1 emerged from single-B-cell cloning and functional screening as a broadly potent RBD-targeting mAb.
- Binding and kinetics: ELISA EC50 values were 0.016 µg/mL (RBD-mFc) and 0.135 µg/mL (S trimer). SPR for Fab-RBD showed ka = 2.55 × 10^5 M−1 s−1, kd = 1.05 × 10−3 s−1, yielding Kd = 0.41 nM, indicating sub-nanomolar affinity.
- ACE2 blockade: 2G1 blocked ACE2 interaction with WT and mutant RBDs with IC50 (µg/mL): WT 0.1504; N439K 0.1050; Y453F 0.2225; E484K 0.1951; N501Y 0.1672. Against S-trimers: WA1/2020 0.0821; Alpha 0.1066; Beta 0.1074; Gamma 0.1047; Delta 0.7973.
- Variant binding affinities (SPR Kd to S trimers, nM): WA1/2020 1.02; Alpha 0.86; Beta 2.77; Gamma 2.03; Delta 15.3. An increased dissociation rate for Delta (kd ≈ 4.27 × 10−2 s−1) contributed to reduced affinity. (Kappa value not provided in the excerpt.)
- Neutralization breadth and potency: • Pseudoviruses: robust neutralization of D614G, Alpha, Beta, Gamma, Delta, Cluster 5; notably IC50 = 0.0005 µg/mL (Gamma) and 0.002 µg/mL (Cluster 5). • Live virus: WA1/2020 IC50 = 0.0240 µg/mL; other VOCs showed decreases to ~0.0138, 0.0046, and 0.0079 µg/mL (values provided without explicit variant mapping in the excerpt); Delta matched WA1/2020 at 0.0240 µg/mL.
- Structural mechanism: Cryo-EM revealed that 2G1 targets a narrow epitope at the tip of RBD with strong hydrophobic interactions; the epitope partially overlaps the ACE2 interface, enabling direct blockade of RBD–ACE2 binding.
- In vivo efficacy and safety: In ACE2 transgenic mice and rhesus macaques challenged with WA1/2020, Beta, or Delta, 2G1 protected against clinical illness and eliminated viral burden without serious safety signals.
- Mutation tolerance: 2G1 maintained blocking against key RBD substitutions (e.g., E484K, N501Y), and mutagenesis suggested resilience to additional/emerging changes near or within the epitope.
The study addresses the urgent need for therapeutics effective against diverse SARS-CoV-2 variants by identifying 2G1, a monoclonal antibody that combines a mutation-tolerant, narrow epitope at the RBD tip with very high affinity, enabling potent neutralization and ACE2 blockade across multiple VOCs. The hydrophobic interaction-dominated interface and partial overlap with the ACE2 binding site provide a mechanistic basis for both potency and breadth. Functional assays demonstrate sub- to low-nanomolar binding and sub-nanomolar to low-ng/mL neutralization potencies against pseudoviruses and live viruses, including variants known for immune escape. In vivo, 2G1 afforded protection and viral clearance in stringent challenge models (100× LD50 in mice; rhesus macaques), with acceptable safety profiles. Together, these findings suggest 2G1’s potential as a therapeutic, as a component of antibody cocktails to mitigate escape, and as a guide for immunogen design focusing on conserved, functionally constrained RBD tip epitopes.
The paper reports the discovery and comprehensive characterization of 2G1, an RBD tip–specific monoclonal antibody with ultra-potent and broad neutralization of SARS-CoV-2 variants, including Alpha, Beta, Gamma, Delta, and Cluster 5. 2G1 exhibits sub-nanomolar binding affinity, effectively blocks ACE2 engagement, neutralizes both pseudoviruses and live viruses at very low concentrations, and confers protection with viral clearance in mouse and macaque models without major safety concerns. Structural insights reveal a hydrophobic, mutation-tolerant epitope overlapping the ACE2 interface, supporting its breadth. These results position 2G1 as a promising candidate for clinical development and as a template for vaccine design and antibody cocktail strategies. Future work should assess clinical efficacy, monitor activity against newer and emerging variants, and further define escape pathways to optimize combination therapies.
Related Publications
Explore these studies to deepen your understanding of the subject.