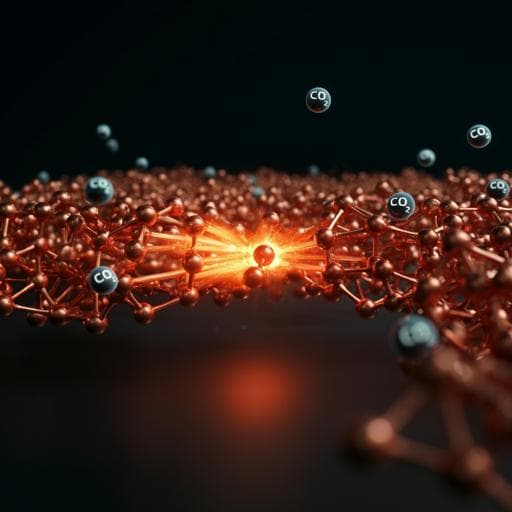
Chemistry
Atomic-scale surface restructuring of copper electrodes under CO₂ electroreduction conditions
R. Amirbeigiarab, J. Tian, et al.
This groundbreaking research conducted by Reihaneh Amirbeigiarab, Jing Tian, Antonia Herzog, Canrong Qiu, Arno Bergmann, Beatriz Roldan Cuenya, and Olaf M. Magnussen explores how potentiodynamic methods can induce structural changes in Cu catalysts, enhancing their selectivity for electrochemical CO₂ reduction. The findings reveal the spontaneous formation of low-coordinated Cu surface species, paving the way for innovative CO₂RR site regeneration.
Related Publications
Explore these studies to deepen your understanding of the subject.