Engineering and Technology
Overtone photothermal microscopy for high-resolution and high-sensitivity vibrational imaging
L. Wang, H. Lin, et al.
The shortwave infrared (SWIR) window (900–2000 nm) offers advantages for bioimaging, including water absorption coefficients that are ~20–5000 times smaller than in the mid-infrared and reduced scattering, enabling larger penetration depths up to millimeters. SWIR spectroscopy provides rich chemical information based on overtone absorption. Existing SWIR imaging approaches (hyperspectral reflectance/transmittance imaging, diffuse optical spectroscopic imaging, and photoacoustic microscopy) face tradeoffs among chemical specificity, sensitivity, spatial resolution, and penetration depth. Visible photothermal and mid-infrared photothermal microscopy provide sensitivity and bond-selectivity, respectively, but visible photothermal often lacks broad chemical specificity, and mid-IR photothermal suffers from strong water absorption, limiting penetration and increasing background in aqueous environments. To address these limitations, the study introduces overtone photothermal (OPT) microscopy: SWIR excitation of C-H overtones coupled with a visible probe to detect thermal lensing. The goal is to achieve high chemical specificity, high sensitivity, and submicrometer resolution while enabling imaging in aqueous and turbid media.
SWIR imaging modalities include: (1) hyperspectral reflectance/transmittance imaging, which provides macroscopic spectral characterization but typically sacrifices spatial resolution due to space-bandwidth limitations; diffraction-limited performance is achievable via magnified FOV or point scanning but often at reduced FOV; (2) diffuse optical spectroscopic imaging (DOSI), a wide-field technique measuring combined absorption and scattering with sub-millimeter lateral resolution and millimeter-scale penetration, useful for thick tissues; (3) photoacoustic microscopy (PAM), which uses optical focusing and ultrasonic detection, demonstrated for imaging lipids (C-H 2nd overtone) and water (1930 nm), though sensitivity depends on ultrasonic transducer quality. Photothermal microscopy comprises visible photothermal (targeting nanoparticles and exploiting field enhancement) and mid-infrared photothermal (MIP) imaging, which provides bond-selective information in the fingerprint region and has been applied to materials and biological systems (e.g., nanowires, perovskites, bacterial metabolism, protein mapping, enzymatic activity). However, MIP is hampered by strong water absorption in aqueous environments and thick tissues. Collectively, these prior methods reveal a gap for a technique that combines high sensitivity, high spatial resolution, chemical specificity in water-rich environments, and reasonable penetration—motivating OPT microscopy.
OPT microscope and signal extraction: A lab-built OPT microscope employs a pulsed SWIR pump (1080–1280 nm) and a 520 nm probe generated by second harmonic generation of a 1040 nm femtosecond laser. Both beams (80 MHz repetition) are chirped to picosecond durations using SF57 glass rods (SWIR: five 15-cm rods; 520 nm: four 15-cm rods) to minimize nonlinear and thermal damage. The pump beam is modulated with an acousto-optic modulator at 100 kHz–1 MHz with duty cycles of 10–50% (typically 50% for biology). The beams are combined collinearly and scanned via 2D galvos on an upright microscope, focused with a 60× water immersion objective (NA 1.2). An adjustable-NA condenser and iris convert thermally induced refractive index gradients into probe intensity modulation via a thermal lens effect. An axial focus offset ΔZ between pump and probe is set using a lens pair to optimize photothermal signal sign and magnitude. The probe intensity modulation is detected by a biased Si photodiode, filtered, amplified (46 dB low-noise preamplifier), and demodulated by a lock-in amplifier; demodulated (AC) signals synchronized to scanning yield OPT images. Typical on-sample powers: SWIR <50 mW; probe <20 mW. Spectral imaging and fidelity: OPT spectra are acquired by tuning the SWIR pump wavelength and recording images; spectral comparisons to UV-Vis-NIR spectrophotometer references validate fidelity (e.g., PS beads, ethylene glycol). Resolution and sensitivity assessment: 200-nm PMMA beads in glycerol-d8 imaged at 1170 nm (C-H 2nd overtone resonance) with modulation at 800 kHz (50% duty, 625 ns pulses) and ~40 mW average pump power. BM3D denoising used for SNR enhancement where indicated. 3D imaging performed by axial stepping (e.g., 200 nm steps) to obtain axial point-spread function and resolution. Thermal modeling: COMSOL simulations model transient heating and cooling for nanoparticles in media (glycerol-d8 vs D2O), providing maximum temperature rise and thermal decay constants to guide modulation frequencies and duty cycles and ensure sufficient heat dissipation. Spectral unmixing (pixel-wise LASSO): Hyperspectral unmixing solves D = C S + E with a local chemical sparsity constraint via LASSO at each pixel: minimize ||D_i - C_i S||_2^2 + β||C_i||_1, using reference spectra S from pure chemicals. References: BSA (protein), triglyceride/TAG (fatty acids), and water (for aqueous media). β is fixed across images. In D2O PBS, two-component unmixing (protein, lipid) is sufficient due to reduced D2O absorption in the 2nd overtone range (~1/50 of H2O). Sample preparations: PS and PMMA blends prepared by dissolving PS and PMMA in toluene and spin-coating onto cleaned coverslips to form phase-separated thin films. PMMA striped nanostructures fabricated by electron-beam lithography on PMMA-coated coverslips, with Au nanoparticle conduction layer, patterning at 30 keV, development (MIBK/IPA) and Au removal; stripe dimensions ~500 nm width, ~650 nm height, 1.5 µm pitch. OVCAR-5 cells cultured in RPMI 1640 with supplements, fixed in neutral buffered formalin, washed in PBS (H2O or D2O), and imaged in a glass-bottom dish with direct water-immersion objective. C. elegans N2 worms maintained on NGM with E. coli OP50, fixed in formalin, washed in PBS (H2O or D2O), and sandwiched between coverslips for imaging. Mouse brain tissue from adult C57BL/6J fixed in formalin, sliced into 200-µm coronal sections, washed in PBS, and imaged in PBS between coverslips. Comparative MIP imaging used a QCL (200 kHz, 500 ns pulses) and 532 nm probe in a reflective objective configuration. Data and code: Hyperspectral stacks acquired by manual SWIR tuning; MATLAB code for LASSO unmixing available at https://github.com/buchenglab.
- Spectral fidelity: OPT spectra of 10-µm polystyrene (PS) beads show peaks at 1144 nm (aromatic C-H 2nd overtone) and 1205 nm (aliphatic C-H 2nd overtone), matching UV-Vis-NIR references. Ethylene glycol shows a broad ~1205 nm peak; overall good agreement with reference spectra.
- Spatial resolution: Imaging 200-nm PMMA beads at 1170 nm yields a lateral FWHM of 432 nm; after deconvolution with 200 nm bead size, lateral resolution is 405 nm. Theoretical diffraction limit (0.61λ/NA) is 242 nm; discrepancy attributed to finite axial offset ΔZ between beams.
- Sensitivity: OPT signal is linear with both SWIR pump and 520 nm probe powers. DMSO in glycerol-d8 detected at 1% concentration with SNR ~8 at 1170 nm; theoretical limit of detection (3σ) ~0.3% DMSO. Single 200-nm PMMA beads resolved with SNR ~4.
- Thermal dynamics: COMSOL simulations for 200-nm PMMA beads indicate maximum temperature rise ~3.6 K in glycerol-d8 (higher than D2O) with thermal decay constants of 35 ns (glycerol-d8) and 28 ns (D2O). Modulation up to 1 MHz feasible with adequate heat dissipation.
- Axial resolution and 3D imaging: Depth-resolved imaging of a single 1-µm PMMA bead (steps of 200 nm) shows an axial resolution of ~2 µm; axial profile depends on ΔZ and interference between scattered and incident fields.
- Materials imaging: Phase-separated PS/PMMA thin films imaged at 1140 nm (PS) and 1170 nm (PMMA) reveal complementary domains; PMMA domains <500 nm are resolved within PS matrix. PMMA stripes (~500 nm width, ~650 nm height, 1.5 µm spacing) are clearly visualized; OPT cross-sections exhibit Gaussian-like profiles consistent with convolution of PSF and sample geometry.
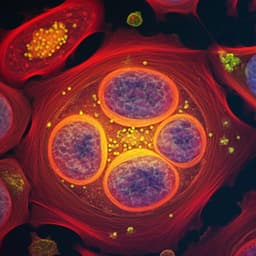
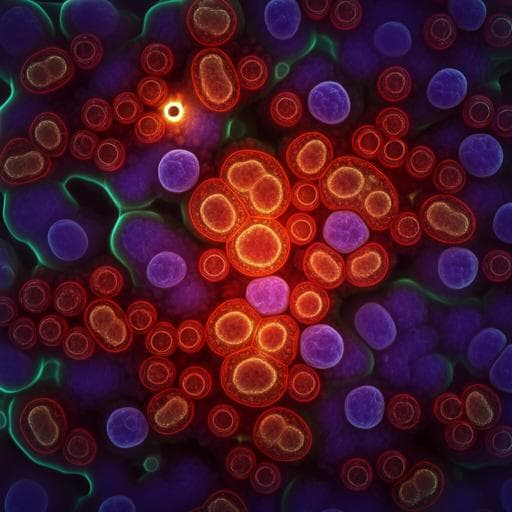
- Biological imaging in aqueous media: Depth-resolved hyperspectral OPT imaging of OVCAR-5 cells enables LASSO-based unmixing of protein, fatty acids, and water in H2O PBS, revealing protein-rich cellular structures and depth-varying lipid droplets; inclusion of water channel removes background and artifacts. In D2O PBS, reduced water background allows two-component unmixing (protein, fatty acids), showing nucleoli, protein networks, and lipid distribution across z-planes.
- Multicellular organisms: In vivo OPT of C. elegans in H2O PBS with three-component unmixing provides protein and lipid maps after removing water background; protein distributed broadly, lipids enriched near intestinal cells and hypodermis; numerous native lipid droplets visualized and 3D variations across tissues (intestine, muscle, hypodermis) observed. Similar specificity confirmed in D2O PBS datasets.
- Brain tissue imaging: In mouse brain slices (200 µm thick) in PBS, depth-resolved OPT at 1190 nm achieves penetration ~100 µm (compared to ~40 µm for MIP in similar tissues). LASSO unmixing at depths (e.g., Z = 10 µm and 30 µm) shows lipid-rich white matter in corpus callosum consistent with high myelin content; individual nerve fibers are resolved; water maps provided. Penetration depth currently limited by visible probe scattering; SWIR beam retains focus over full objective working distance (~0.28 mm).
- Throughput: A 400×400 pixel OPT image acquisition ~3 s; manual wavelength tuning ~5 s per change, currently limiting hyperspectral speed.
OPT microscopy combines SWIR excitation of overtone bands with a visible probe to achieve high chemical specificity, sensitivity, and submicrometer spatial resolution in aqueous and turbid environments. Relative to existing SWIR imaging approaches that trade resolution for FOV and penetration, OPT achieves 405 nm lateral resolution and detects 200-nm particles while maintaining overtone-based chemical contrast. Compared to mid-IR photothermal (MIP) imaging, OPT avoids strong water absorption and is compatible with high-NA refractive objectives (e.g., NA 1.2 water immersion), boosting signal strength and simplifying collinear scanning alignment. The pump-probe nature confers optical sectioning and mitigates backgrounds from one-photon scattering, beneficial in turbid tissues. LASSO-based spectral unmixing, leveraging local chemical sparsity, effectively disentangles overlapping overtone spectra to produce protein, lipid, and water maps across depths in cells, C. elegans, and brain tissue. Current depth performance (~100 µm) is limited primarily by visible probe scattering; moving to longer-wavelength probes could enhance penetration while preserving sectioning. Quantification can be biased by local scattering since photothermal signals scale with both absorption and scattering; incorporating water as a calibration channel or adopting phase-only detection could mitigate this. Broad overtone peaks demand sufficient SNR; optimized band selection could reduce required spectral frames and accelerate hyperspectral imaging. Overall, OPT expands SWIR spectroscopy to high-resolution, high-sensitivity chemical imaging at cellular to tissue scales.
The study introduces overtone photothermal (OPT) microscopy, a label-free, bond-selective imaging modality that bridges visible and mid-infrared photothermal techniques by exploiting SWIR overtone excitation and visible probe detection. OPT achieves 405 nm lateral resolution, detects nanoparticles (200-nm PMMA), demonstrates spectral fidelity to NIR references, and attains sensitive detection of dilute solutes (down to 0.3% DMSO theoretical). It enables depth-resolved chemical mapping of polymers, intracellular protein and lipid distributions in cancer cells, multicellular organization in C. elegans, and lipid-rich structures in mouse brain white matter with penetration to ~100 µm in turbid tissue. LASSO-based unmixing improves specificity amid overlapping overtone bands and removes water backgrounds, yielding clear protein and lipid maps. Future directions include enhancing depth by using longer-wavelength probes, increasing hyperspectral speed with automated fast tuning and optimized band selection, integrating epi detection for opaque or backscattering samples, expanding spectral coverage (e.g., into 1st overtones with broadband sources), and deploying multimodal imaging on the same ultrafast platform. These advances will facilitate multiscale chemical and structural investigations in materials and biology.
- Penetration depth is currently limited to ~100 µm in turbid tissues due to visible probe scattering and wavefront distortion; the SWIR pump is less limiting.
- Overlapping overtone bands in the SWIR region cause spectral crosstalk, necessitating robust unmixing and high SNR.
- Photothermal signal depends on both absorption and local scattering, potentially biasing absolute concentration estimates; current chemical maps are semi-quantitative (relative concentrations).
- Broad spectral features require high SNR and many spectral frames; current manual wavelength tuning (~5 s per change) slows hyperspectral acquisition.
- Current implementation is in transmission mode; epi detection is not yet integrated, limiting applicability to opaque samples or strong backscatterers.
- Achieved lateral resolution (405 nm) is above the theoretical diffraction limit (242 nm) due to practical axial focus offsets (ΔZ) between pump and probe.
Related Publications
Explore these studies to deepen your understanding of the subject.