Psychology
Distinct patterns of connectivity with the motor cortex reflect different components of sensorimotor learning
C. N. Areshenkoff, A. J. D. Brouwer, et al.
The study addresses how distinct implicit and explicit processes jointly support sensorimotor learning and how their neural substrates can be disentangled from performance-related signals. Historically, motor learning was viewed as a unitary implicit process, but behavioral and computational work demonstrates parallel slow implicit and fast explicit systems. Implicit learning is linked to updating internal models for sensory-guided control (cerebellar and sensorimotor networks), whereas explicit learning likely recruits higher-order association cortex. Because performance reflects the combined output of both processes and may be confounded by error-related feedback and attentional control, the authors aim to isolate neural patterns specifically associated with implicit adaptation, explicit strategy use, and general performance during a visuomotor rotation task, and test whether these neural signatures generalize to a separate reward-based motor learning paradigm that relies primarily on explicit mechanisms.
Prior work frames implicit sensorimotor learning within optimal feedback control and internal model updating supported by cerebellum and sensorimotor cortex. The neural basis of explicit learning is less defined and may involve association and prefrontal regions, with studies showing increased lateral prefrontal activity during early adaptation. Explicit strategies such as mental rotation correlate with longer response times and rapid error reduction. However, neural activity attributed to explicit processes may reflect improved performance or reduced error feedback rather than explicit learning per se. The principal gradient of cortical organization—from unimodal to transmodal cortex (DMN)—provides a hierarchical framework to situate these processes. Behavioral evidence shows explicit processes dominate in tasks without sensory prediction errors, such as reinforcement-based motor learning, requiring credit assignment and strategy formation over longer time horizons.
Participants: Forty-six right-handed adults were recruited; 36 were included after exclusions for motion, task interruption, poor task performance, or failure to follow instructions. Testing comprised three sessions: an MRI-training session and two MRI experimental sessions.
Tasks and Procedure: Subjects performed two motor learning tasks in the MRI: (1) a visuomotor rotation (VMR) sensorimotor adaptation task and (2) a reward-based motor learning task. In the VMR task, subjects performed 64 baseline trials with veridical cursor feedback, followed by 160 learning trials with a 45° clockwise cursor rotation, requiring a counterclockwise re-aiming to hit targets. After learning, 16 report trials measured explicit aiming strategy using a joystick-controlled line; the final 8 report trials were used to estimate explicit strategy to minimize initial confusion effects. Implicit learning was defined as the mean difference between reported and actual aim direction; explicit learning as the mean difference between reported aim and target direction. Early performance was quantified as mean error during the first 100 imaging volumes (~32 trials). A separate behavioral control study (N=14) interleaved report trials throughout learning to verify that late reports reflect early explicit strategy (r=0.77, p=0.0012).
Reward-based task: Subjects traced a visible rightward-curved path without cursor feedback; during learning (200 trials), scalar scores (0–100) were based on similarity to a hidden mirror-image path (not disclosed), preventing error-based learning. A generalized sigmoid was fit to a lateral movement score per trial to segment pre-learning, learning, and post-learning periods and derive learning rate (k). Fits used Bayesian regularization via HMC (Stan), with model checks confirming convergence.
MRI Acquisition and Preprocessing: Scans were acquired on a 3T Siemens Trio with standard EPI parameters (TR=2s, TE=30ms), whole-brain coverage, and sessions including structural T1w/T2w images, DTI, resting-state, and task fMRI. Preprocessing used fMRIPrep 1.4.1 with skull stripping, tissue segmentation, normalization to MNI spaces, motion correction, slice-time correction, ICA-AROMA denoising, CompCor regressors, and confound regression. ROI time series were extracted from: cortex (Schaefer 400-parcel atlas with 17 networks, focusing on Somatomotor hand areas for motor cortex seeds), cerebellum (Diedrichsen atlas), and basal ganglia (Harvard-Oxford).
Connectivity Estimation and Centering: For each subject and epoch (baseline, early learning, late learning for VMR; pre-learning, learning, post-learning for reward task), covariance matrices (Ledoit–Wolf shrinkage) were computed for 100-volume windows. To reduce subject-specific static covariance and enhance task-related variance, covariance matrices were centered via a Riemannian approach: projection to tangent space at subject mean and parallel transport to the grand mean across sessions.
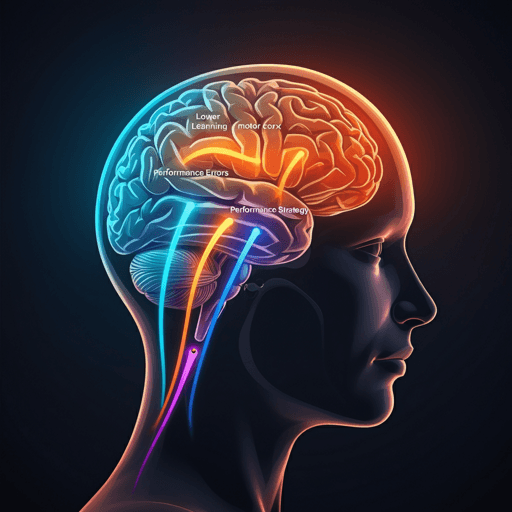
Predictive Modeling and Neural Axes: Whole-brain functional connectivity was computed between the contralateral (left) hand area of primary motor cortex and all ROIs. To isolate learning-specific processes from general performance, explicit and implicit behavioral measures and ROI connectivities were orthogonalized by regressing out early error (mean early learning error), using residuals. Group-regularized ridge regression (GRridge) with network-specific penalties (tuned by leave-one-out CV) predicted three behavioral measures from connectivity during early learning: (1) Explicit learning, (2) Performance (inverted error, so higher denotes better performance), and (3) Implicit learning. The resulting coefficient vectors define three neural axes—Explicit, Performance, Implicit—indicating ROIs whose connectivity with motor cortex relates to each component.
Generalization Analysis: For the reward-based task, axis expression scores per subject were computed as the spatial correlation between each VMR-derived neural axis and the subject’s connectivity pattern with motor cortex in each period (pre-learning, learning, post-learning). Correlations between axis expression changes (especially during learning) and reward-task learning rates were tested. Control analyses reconstructed axes using ipsilateral hand and tongue motor areas and late VMR epochs to assess specificity.
- Behavioral validation: Explicit reports correlated with response times during both early (r=0.45, p=0.006) and late learning (r=0.69, p=2.57e−06), consistent with time-consuming explicit strategies (e.g., mental rotation). Explicit learners showed decreasing error with longer RTs, unlike implicit learners (mean within-subject Spearman correlation between error and RT: explicit m_exp = −0.44 vs implicit m_imp = 0.11; t34 = −2.38, p = 0.033).
- Estimation windows: Early performance defined over the first ~32 trials (100 volumes) captured the period when explicit strategies emerge; a behavioral control study confirmed that late explicit reports reflect early strategies (r = 0.77, p = 0.0012).
- Neural axes (early learning, contralateral hand area; cross-validated R²): Explicit R² = 0.29; Performance R² = 0.82; Implicit R² = 0.64.
- Axis topographies:
- Implicit axis: Highest loadings in the dorsal attention network (DAN-B), including superior parietal and premotor regions; cerebellar motor regions showed alignment to the implicit axis in targeted analyses.
- Performance axis: Highest loadings in frontoparietal Control-A subnetwork (DLPFC, IPL, anterior cingulate), consistent with attention, monitoring, and error-detection processes.
- Explicit axis: Highest loadings in default mode network (DMN-B), including middle/inferior frontal gyrus, angular gyrus, and anterior/middle temporal cortex.
- Cross-loading observation: Control-A and Control-C subnets exhibited mixed loadings, with PCC/precuneus particularly explicit-aligned.
- Cortical hierarchy: An explicit-alignment summary axis (explicit minus implicit rank) correlated with the principal cortical gradient (unimodal to transmodal; spin test p = 0.028), placing implicit axis near sensorimotor regions and explicit axis at the apex (DMN).
- Across-task generalization (reward-based learning): Increased expression of the VMR-derived Explicit axis during the learning period predicted faster reward-based learning (r = 0.34, t34 = 2.04, p = 0.049). No such relationship for Implicit (r = −0.12, p = 0.48) or Performance axes (r = −0.05, p = 0.77), nor during pre-learning (r = −0.06, p = 0.74) or post-learning (r = −0.18, p = 0.32).
- Specificity controls:
- Motor-area specificity: Reconstructing axes using ipsilateral hand or contra/ipsilateral tongue motor areas abolished the correlation with reward-task learning.
- Epoch specificity: Axes derived from late VMR learning did not predict reward-task learning; late explicit axis shifted to DAN/visual regions (premotor/posterior parietal/visual), consistent with strategy implementation (e.g., mental rotation) rather than strategy learning.
- Cerebellar connectivity: Direct cerebello–motor connectivity analyses showed greatest task-related modulation in anterior motor cerebellum, aligning with known roles in sensory prediction error computation.
The study disentangles three concurrent components of sensorimotor adaptation—implicit learning, explicit strategy formation, and performance-related processes—by examining how whole-brain connectivity with motor cortex changes during learning. Implicit adaptation aligns with sensorimotor-adjacent DAN regions (superior parietal and premotor), consistent with internal model updating and state estimation within an optimal feedback control framework. Performance-related connectivity emphasizes frontoparietal control areas (DLPFC, IPL, ACC), likely reflecting general task demands such as spatial attention, performance monitoring, and error detection that benefit both learners. Crucially, explicit learning corresponds to connectivity with transmodal DMN nodes positioned at the apex of the cortical hierarchy, supporting internally driven, perceptually independent computations over longer time horizons (e.g., forming task structure representations and rule-sets). The explicit-alignment axis’s correlation with the principal gradient reinforces a hierarchical organization wherein implicit processes are proximal to sensorimotor systems and explicit processes depend on high-level integrative networks. Generalization to a reward-based task without sensory prediction errors demonstrates that only the Explicit axis predicts learning rate, underscoring a role for DMN-mediated explicit processes in strategy discovery and credit assignment when detailed error information is unavailable. These results clarify long-standing ambiguities around prefrontal and control network involvement—suggesting their contributions are more related to performance support than explicit strategy formation—and highlight the DMN’s relevance to explicit motor learning, in line with broader literature on self-generated cognition and strategy formation.
This work establishes a tripartite mapping between motor-cortex connectivity patterns and the distinct components of sensorimotor learning: an Implicit axis (DAN/parietal-premotor), a Performance axis (frontoparietal control), and an Explicit axis (DMN/transmodal cortex). The axes occupy ascending levels of the cortical processing hierarchy, with explicit learning at the apex, and only the Explicit axis generalizes to predict learning in a separate reward-based task that relies on explicit processes. These findings advance mechanistic understanding of how different neural systems cooperate during adaptation and strategy formation and provide a framework for studying generalization across motor learning contexts. Future directions include: extending analyses beyond motor-cortex-centered bipartite interactions to chart network–network dynamics; refining the roles of cerebello–striatal loops using methods sensitive to weaker cortico–subcortical connectivity; probing temporal receptive windows and memory systems underpinning explicit strategy formation; and testing causal contributions of DMN and control subsystems via perturbation or longitudinal designs.
- Connectivity focus: Analyses were restricted to bipartite functional interactions between primary motor cortex and other regions, limiting inferences about inter-regional connectivity outside motor cortex.
- Subcortical sensitivities: Cerebellar and striatal regions loaded more weakly than cortical regions, likely due to lower cortico–subcortical functional connectivity strength and reduced predictive utility in regression; targeted analyses confirmed expected cerebellar motor involvement.
- Reporting design: Explicit reports were collected post-learning to avoid biasing learning; a separate behavioral study validated their correspondence to early strategy, but trial-wise explicit dynamics during early learning were not directly measured in MRI.
- Task order: Reward-based task preceded VMR in all subjects to maintain naivete; lack of counterbalancing may introduce order effects, though it strengthens across-task prediction interpretations.
- Strategy specificity: Late-learning explicit axis appeared to reflect strategy implementation (e.g., mental rotation) rather than learning per se, indicating epoch-dependent differences in explicit processes not exhaustively characterized here.
- Generalizability scope: Findings pertain to right-handed young adults and two task paradigms; broader populations and diverse motor tasks warrant investigation.
Related Publications
Explore these studies to deepen your understanding of the subject.