Medicine and Health
A therapeutic neutralizing antibody targeting receptor binding domain of SARS-CoV-2 spike protein
C. Kim, D. Ryu, et al.
The study addresses the urgent need for effective therapeutics against COVID-19 by identifying and characterizing a human monoclonal antibody that targets the SARS-CoV-2 spike protein receptor binding domain (RBD). Neutralizing antibodies are promising due to their potential to block viral entry by interfering with ACE2 interaction and to be used in combination with antivirals such as remdesivir. Convalescent plasma presents practical limitations (scalability, variability), motivating monoclonal antibody development. SARS-CoV-2 uses the spike protein to bind ACE2 with higher affinity than SARS-CoV-1; variants such as D614G have increased transmission and dominate globally. The RBD is a critical determinant of viral attachment and a main target of neutralizing antibodies with minimal risk of antibody-dependent enhancement (ADE). The authors aimed to discover, structurally define, and evaluate in vivo a potent RBD-targeting antibody (CT-P59) that blocks ACE2 binding and suppresses viral replication.
Background work established that SARS-CoV-2 spike engages ACE2 via the RBD with higher affinity than SARS-CoV-1, correlating with efficient transmission. The D614G spike variant enhances infectivity and transmission. Prior structural studies have identified multiple neutralizing antibodies targeting RBD, many derived from IGHV3 germlines, that block ACE2 by binding the receptor-binding motif (RBM). Cryo-EM studies revealed RBD 'up' and 'down' conformations, with ACE2 engaging only the 'up' state. Concerns over ADE in coronavirus infections have generally been minimal for RBD-targeting antibodies. This study situates CT-P59 among known neutralizing mAbs but highlights that its binding orientation and epitope are distinct from many previously reported anti-RBD antibodies.
Antibody discovery: Peripheral blood mononuclear cells (PBMCs) were isolated from a convalescent COVID-19 patient (IRB-approved). RNA was extracted, cDNA synthesized, and antibody variable regions (VH and VL) amplified. A phage display single-chain variable fragment (scFv) library was constructed in pCombs3x5 and subjected to iterative biopanning against recombinant SARS-CoV-2 RBD. RBD-specific binders were identified by plaque ELISA and advanced for characterization. Expression and purification: Selected scFvs were expressed in CHO cells; full-length IgG constructs for candidate antibodies (including CT-P59) were generated by cloning synthesized heavy and light chain genes into mammalian expression vectors and expressing in CHO cells. Recombinant SARS-CoV-2 RBD and spike proteins were produced and purified (His-tag, Ni-NTA; Protein A for antibodies). Binding and neutralization assays: Neutralization of authentic SARS-CoV-2 (including wild type and D614G variant) was assessed in VeroE6 cells via plaque reduction and TCID50-based assays to determine IC50 values and viral titers post-treatment. Affinity measurements were performed by surface plasmon resonance (SPR, Biacore T200) using RBD immobilized on CM5, and by biolayer interferometry (BLI, Octet) to assess ACE2-competition and binding to mutant RBDs. CT-P59 exhibited high affinity to RBD (Kd 27.9 pM by BLI/SPR). Structural biology: The CT-P59 Fab was complexed with SARS-CoV-2 RBD (1:2 molar ratio), purified by size exclusion chromatography, and crystallized by sitting-drop vapor diffusion. X-ray diffraction data were collected at beamline BL-8C (Pohang Light Source). The complex structure was solved by molecular replacement and refined to 2.7 Å resolution. Structural comparisons were made to ACE2-bound RBD (PDB 6LZG) and to other RBD–antibody complexes using PISA for interface analysis. ADE assessment: In vitro ADE assays were conducted in permissive VeroE6 cells and Fc receptor-bearing cell lines (e.g., Raji, U937). SARS-CoV-2 was incubated with a range of antibody concentrations (CT-P59, controls CR3022 and CT-P27) and infection quantified by nucleocapsid-specific readouts; no enhancement was observed. Animal models: Three in vivo challenge models were used. Ferrets were infected intranasally and intratracheally with SARS-CoV-2 and treated intravenously with CT-P59 at 1 day post-infection (doses 3 mg/kg and 30 mg/kg) or controls; a remdesivir comparator arm (18 mg/kg daily for 5 days) was included. Viral titers (nasal wash, lungs) were measured by TCID50 and qRT-PCR; rectal swabs assessed by qRT-PCR. Golden Syrian hamsters were infected intranasally and treated intraperitoneally with CT-P59 (15, 30, 60, 90 mg/kg) 24 h post-challenge; lungs were harvested at 5 dpi for viral titration and RNA quantification. Rhesus macaques were infected and monitored for viral loads in upper airways (nose, throat) by TCID50 and RNA copies, and for clinical signs (fever, weight loss, respiratory symptoms). Statistical analyses used two-way ANOVA with Dunnett’s test for group comparisons.
- Potent neutralization: CT-P59 effectively neutralized authentic SARS-CoV-2, including the D614G variant, in VeroE6 cells, blocking RBD–ACE2 interaction and reducing plaque formation.
- High-affinity binding: CT-P59 bound SARS-CoV-2 RBD with a dissociation constant Kd of 27.9 pM.
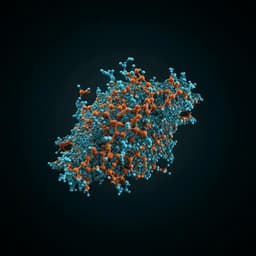
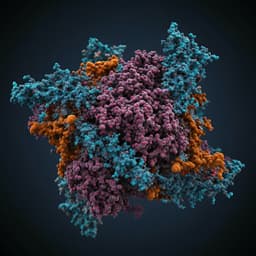
- Structural mechanism: The 2.7 Å crystal structure of CT-P59 Fab in complex with RBD showed binding within the RBM and substantial overlap with the ACE2-binding surface. The RBD conformation remained largely unchanged upon binding (RMSD 0.89 Å over 193 Cα atoms), with a local change in the β5–β6 loop (residues 473–488). The interface is dominated by the heavy chain (∼825 Ų SASA) with minor contribution from the light chain (∼113 Ų). Sixteen heavy-chain residues contact 19 RBD residues within 4.5 Å; the CDR H3 (18 residues, α-helical) mediates key hydrogen bonds and hydrophobic interactions. Of 21 RBD residues contacting CT-P59, 12 overlap with ACE2-contact residues, indicating direct ACE2-blockade.
- Distinct epitope/orientation: CT-P59’s binding orientation differs from many reported IGHV3-derived anti-RBD antibodies; it shares partial epitope overlap with different antibody classes and can bind RBD in the spike ‘up’ conformation without steric clashes.
- In vivo efficacy (ferrets): Intravenous CT-P59 at 30 mg/kg significantly reduced viral RNA in nasal wash by 2 dpi and eliminated detectable infectious virus in nasal wash by 6 dpi; lung infectious titers were significantly reduced by 3 dpi and undetectable by 7 dpi at 30 mg/kg. The 3 mg/kg dose also reduced viral RNA from 4 dpi. Rectal swab RNA copies decreased from 4 dpi at both doses. Clinical symptoms and lung pathology improved concordantly. Compared with remdesivir (18 mg/kg daily for 5 days), CT-P59 achieved earlier clearance; remdesivir-treated ferrets still had detectable infectious virus in nasal wash at 6 dpi.
- In vivo efficacy (hamsters): CT-P59 administered 24 h post-infection reduced lung viral RNA at 5 dpi across all doses (15–90 mg/kg). While control lung titers peaked at 3 dpi (8.3 log TCID50) and declined to 6.8 log TCID50 by 5 dpi, CT-P59 at 15 mg/kg significantly attenuated titers; doses 30–90 mg/kg yielded no detectable infectious virus in lungs 48 h after treatment, indicating complete suppression of replication.
- In vivo observations (rhesus macaques): Viral loads peaked at 2 dpi (approximately 4.3 and 3.2 log TCID50 in assessed samples), with CT-P59-treated animals showing reduced titers in upper airways; no notable clinical manifestations were observed in either treated or control animals.
- Safety regarding ADE: No antibody-dependent enhancement was detected in vitro in FcR-bearing cells or permissive cells across a broad range of CT-P59 concentrations, consistent with absence of worsened symptoms in treated animals.
The findings demonstrate that CT-P59 directly blocks ACE2 engagement by occupying overlapping RBM residues on the RBD, providing a clear mechanistic basis for neutralization. Its distinct binding orientation and epitope relative to many IGHV3-derived antibodies expand the repertoire of ACE2-blocking epitopes and may offer complementary coverage against viral escape. Across three animal models, CT-P59 rapidly reduced viral titers in both upper and lower respiratory tracts and alleviated clinical signs and lung pathology, with faster viral clearance than remdesivir in ferrets. The absence of ADE in vitro and lack of adverse clinical signals in animals support a favorable safety profile. Collectively, the data indicate CT-P59 is a strong therapeutic candidate for treatment, and potentially prophylaxis, of COVID-19, including against the prevalent D614G variant.
This work identifies CT-P59, a human monoclonal antibody targeting the SARS-CoV-2 RBD, and elucidates its high-affinity, ACE2-blocking mechanism via a 2.7 Å crystal structure. CT-P59 exhibits potent in vitro neutralization and robust in vivo efficacy in ferret, hamster, and rhesus macaque models, with no evidence of ADE. Compared with remdesivir in ferrets, CT-P59 achieved earlier viral clearance in the upper airways. These results support CT-P59 as a promising therapeutic for COVID-19. Future work should include clinical trials to assess safety and efficacy in humans, evaluation in combination therapies, and continued monitoring of breadth against emerging variants.
- The efficacy data are limited to in vitro assays and animal models; no human clinical outcomes are reported.
- Sample sizes in animal cohorts are modest, and clinical signs in rhesus macaques were minimal, limiting assessment of symptom improvement.
- While effective against the D614G variant, breadth against a wider range of emerging variants was not comprehensively assessed here.
- Detailed pharmacokinetics, dosing optimization, and long-term safety were not addressed in this study.
Related Publications
Explore these studies to deepen your understanding of the subject.